“The periodic table is a tabular method of displaying the elements in such a way, that the elements having similar properties occur in the same vertical column or group”.
Early Models of Periodic Table
Dobereiner’s Triads
Dobereiner arranged a group of three elements with similar properties in the order of increasing atomic masses and called it a triad. He showed that the atomic mass of the middle element is approximately the arithmetic mean of the other two.
For Example Li (6.9), Na (23), K (39).
Limitation: It fails to arrange all the known elements in the form of triads, even having similar properties.
Newland’s Law of Octaves
According to this ‘when elements are placed in order of increasing atomic masses, the physical and chemical properties of every 8th element are a repetition of the properties of the first element.’
Limitations
- Law of octaves was applicable only upto calcium (only for lighter elements).
- Newland adjusted two elements in the same slot (e.g. Co and Ni), having different properties. For example; Co and Ni with Fluorine, Chlorine, Bromine and Iodine.
- According to Newland, only 56 elements existed in nature and no more elements would be discovered in future.
Present attempts for the classification of elements: Mendeleev’s Periodic Table, the Modern Periodic Table.
Mendeleev’s Periodic Table
Mendeleev’s periodic table is based on the physical and chemical properties of elements and their atomic masses.
Mendeleev’s Periodic Law: According to this “The physical and chemical properties of the elements are the periodic function of their atomic masses.”
Periodicity of Properties: The repetition of properties of elements after certain regular intervals is known as Periodicity of Properties.
Merits of Mendeleev’s Periodic Table
- Mendeleev’s left vacant places in his table which provided an idea for the discovery of new elements. Example: Eka-boron, Eka-aluminium and Eka-silicon.
- Mendeleev’s periodic table was predicted properties of several undiscovered elements on the basis of their position in Mendeleev’s periodic table.
- It is useful in correcting the doubtful atomic masses of some elements.
- Noble gases could accommodate in the Mendeleev’s periodic table without disturbing the periodic table after discovery.
Limitations of Mendeleev’s Periodic Table
1. Position of hydrogen: Hydrogen resembles both, the alkali metals (IA) and the halogens (VIIA) in properties, so, Mendeleev could not justify its position.
2. Position of isotopes: Atomic weight of isotopes differ, but, they were not placed in different positions in Mendeleev’s periodic table.
3. Anomalous pairs of elements: Cobalt (Co) has higher atomic weights but was placed before Nickel (Ni) in the periodic table.
4. Placement of like elements in different groups: Platinum (Pt) and Gold (Au) has similar properties but were placed in different groups.
5. Cause of periodicity: He could not explain the cause of periodicity among the elements.
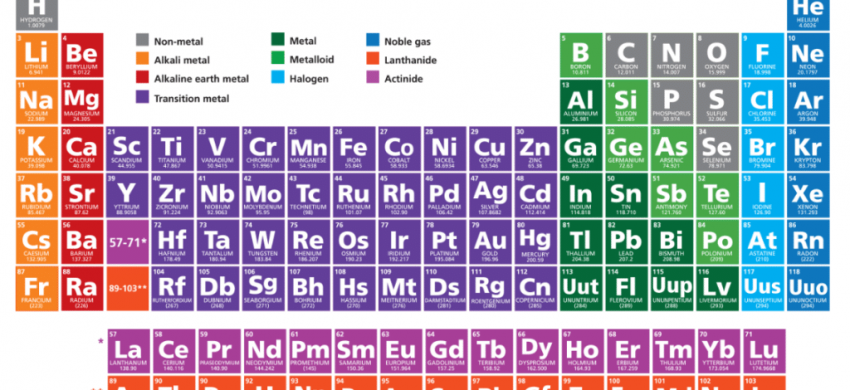
Modern Periodic Law
The physical and chemical properties of elements are the periodic function of their atomic numbers.
Cause of periodicity – It is due to the repetition of same outer shell electronic configuration at a certain regular interval.
Periods in Modern Periodic Table
Elements present in the same period have the same number of shells which is equal to the period number.
On moving from left to right in a given period, the number of electrons in the valence shell increases from one to eight while the number of shells remains the same.
Number of Elements in a Period
The first period contains only two elements 1Hand2He and is known as the shortest period.
The second period (3Lito10Ne) and the third period (11Na to 18Ar) contain 8 elements each and are known as short periods.
The fourth period (19K to 36Kr) and the fifth period (37Rb to 54Xe) contain 18 elements each and are called long periods.
The sixth period contains 32 elements (55Cs and 86Rn) and is also known as the longest period.
The seventh period is an incomplete period.
(After the recent discoveries of the new elements and their addition to the periodic table, the seventh period is officially complete)
Groups in Modern Periodic Table
The modern periodic table contains 18 vertical columns known as groups.
Group 1 elements are known as alkali metals.
Group 2 elements are known as alkaline earth metals.
Group 15 elements are known as pnicogens.
Group 16 elements are known as chalcogens.
Group 17 elements are known as halogens.
Group 18 elements are known as noble gases.
Alkali Metals
The elements in the first group, lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr) are called alkali metals.
They were given the name because they all react with water to form alkalis.
The alkali metals are all shiny, soft, highly reactive solids at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1.
Number of valence electrons = 1
Alkali Earth Metals
Halogens
Noble Gases
Classification of Modern Periodic Table
Metals
Nonmetals
Metalloids
Trends in the Modern Periodic Table
Trends in Modern Periodic Table
Variation of Valency
Variation of Atomic Size
Atomic size or radii: It is defined as the distance from the centre o the nucleus to the valence shell of the atom.
Along the period – Atomic radius decreases because effective nuclear charge increases by one unit and it pulls valence electrons or the electron cloud closer to the nucleus.
Down the group – Atomic radius increases because new shells are added, hence, the distance between the nucleus and valence electrons or the electron cloud increases.
Variation of Metallic Properties
Along the period – Metallic character decreases because the tendency to lose valence electrons decreases due to increasing nuclear charge.
Down the group- As the distance between the nucleus and outermost electron increases, nuclear pull decreases. This increases the tendency of an atom to lose valence electron/s, hence metallic character increases.
Variation of Nonmetallic Properties
Along the period – Non-metallic character increases as the tendency to gain electrons in the valence shell increases due to increasing nuclear charge.
Down the group – As the distance between the nucleus and valence shell increases, nuclear pull decreases. This decreases the tendency of an atom to gain an electron its valence shell, hence non-metallic character decreases.
Variation of Electronegativity
Along the period – Electronegativity increases as the tendency to gain electrons in the valence shell increases due to increasing nuclear charge.
Down the group – As the distance between the nucleus and valence shell increases, nuclear pull decreases. This decreases the tendency of an atom to gain an electron, hence electronegativity decreases.


