NCERT Solutions Class 9 Science Chapter 4 Structure Of The Atom
Page: 47
- What are the canal rays?
Solution:
The radiations that are positively charged are canal rays. This discovery was crucial in the discovery
of another subatomic particle that was positively charged – proton.
- If an atom contains one electron and one proton, will it carry any charge or not?
Solution:
Since a proton is a positively charged particle and an electron is a negatively charged particle, the net
charge becomes neutral as both the particles neutralizes each other.
Page: 49
- On the basis of Thompson’s model of an atom, explain how the atom is neutral as a whole.
Solution:
As per Thompson’s model of an atom,
(i) An atom contains a positively charged sphere in which the negatively charged electrons are implanted.
(ii) Electrons and protons are equal in magnitude, hence an atom on the whole is electrically neutral.
- On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Solution:
As per Rutherford’s model of an atom, the positively charged protons are the ones that are present in the atom.
- Draw a sketch of Bohr’s model of an atom with three shells.
Solution:
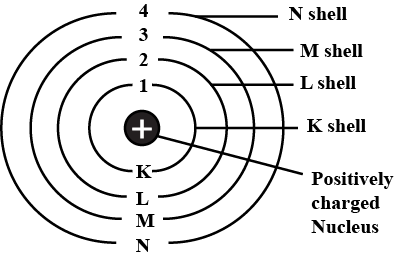
Page: 49
- Name the three subatomic particles of an atom.
Solution:
An atom consists of three subatomic particles:
- Protons – positively charged
- Electrons – negatively charged
- Neutrons – neutral in nature ( no charge )
- Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Solution:
Given: Atomic mass of helium atom = 4u, 2 protons in helium nucleus
Atomic mass = number of protons + number of neutrons
4 = 2 + number of neutrons
Number of neutrons = 4 – 2 = 2e
Helium has 2 neutrons.
Page: 50
- Write the distribution of electrons in Carbon and Sodium atoms.
Solution:
Distribution of electrons in Carbon atoms:
The atomic number of Carbon is 6
Number of electrons is equal to the number of protons in carbon atom i.e., 6
The distribution of electrons in carbon atom is K – 2, L – 4
Distribution of electrons in sodium atoms:
The atomic number of Sodium is 11
Number of electrons is equal to the number of protons in sodium atom i.e., 11
The distribution of electrons in sodium atom is K – 2, L – 8, M – 1
- If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Solution:
K shell can hold 2 electrons
L shell can hold 8 electrons
Hence, when both the shells are full, the total number of electrons present in the atom = 2+8 = 10 electrons.
Page: 52
- How will you find the valency of chlorine, sulphur and magnesium?
Solution:
The definite combining capacity of the atoms of each element, wherein electrons are lost, gained or shared to make the octet of electrons present in the outermost shell is defined as valency. To measure valency, we can figure out the number of electrons that are required to complete the shell in which it is contained or losing excess electrons if present, once the filling is complete.
To find the valency of chlorine:
The atomic number of chlorine is 17
Number of electrons is equal to the number of protons in chlorine i.e., 17
The distribution of electrons in chlorine atom is K – 2, L – 8, M – 7
Hence, from the distribution of chlorine it is clearly evident that to fill the M shell only one electron is required. Therefore its valency is -1. i.e, one electron less
To find the valency of sulphur:
The atomic number of sulphur is 16
Number of electrons is equal to the number of protons in sulphur i.e., 16
The distribution of electrons in sulphur atom is K – 2, L – 8, M – 6
Hence, from the distribution of sulphur it is clearly evident that to fill the M shell two more electrons are required. Therefore its valency is -2, i.e., two electrons lesser.
To find the valency of magnesium:
The atomic number of magnesium is 12
Number of electrons is equal to the number of protons in magnesium i.e., 12
The distribution of electrons in magnesium atom is K – 2, L – 8, M – 2
Hence, from the distribution of magnesium it is clearly evident that to fill the M shell six more electrons are required. But M shell has two electrons only. It possesses lesser electrons than needed to fill the shell.
Thus, we say that the magnesium atom is not stable as the M shell has 2 electrons. Its valency is +2, meaning it has 2 electrons in excess.
- Find out the mass number of oxygen and sulphur atom.
Solution:
(a) To find the mass number of Oxygen:
Number of protons = 8
Number of neutrons = 8
Atomic number = 8
Atomic mass number = Number of protons + number of neutrons = 8 + 8 = 16
Therefore, mass number of oxygen = 16
(b) To find the mass number of Sulphur:
Number of protons = 16
Number of neutrons = 16
Atomic number = 16
Atomic mass number = Number of protons + number of neutrons = 16 + 16 = 32
Questions from NCERT Text Book
Question 1. Compare the properties of electrons, protons and neutrons.
Answer:
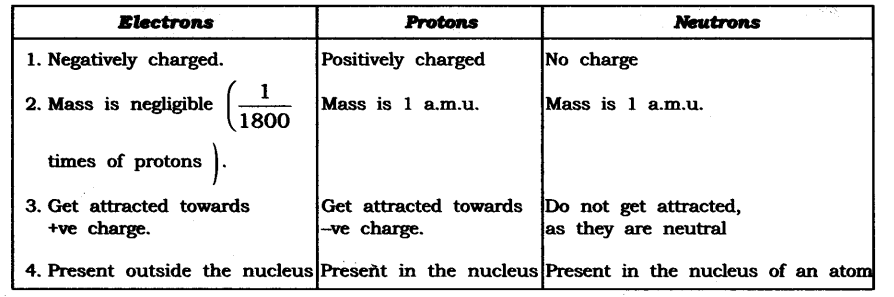
Question 2. What are the limitations of J.J. Thomson’s model of the atom?
Answer: According to J.J. Thomson’s model of an atom, the electrons are embedded all over in the positively charged spheres. But experiments done by other scientists showed that protons are present only in the centre of the atom and electrons are distributed around it.
Question 3. What are the limitations of Rutherford’s model of the atom?
Solution:
Following are the limitations of Rutherford’s model of the atom:
- There is no expected stability in the revolution of the electron in a circular orbit
- Charged particles radiate energy when accelerated thus causing the revolving electrons to lose energy and would fall into the nucleus
- Hence atoms must be highly unstable. Matter would not exist in their known form which clearly is an assumption as atoms are highly stable
Question 4. Describe Bohr’s model of the atom.
Solution:
- An atom holds the nucleus at the centre.
- Negatively charged electrons revolve around the nucleus.
- The atoms in it contains distinct orbits of electrons.
- Electrons do not radiate energy when they are in their orbits.
- The distinct orbits are named as K, L, M, N orbits. Numbers used to denote them are n=1, 2, 3, 4

Question 5. Compare all the proposed models of an atom given in this chapter.
Solution:
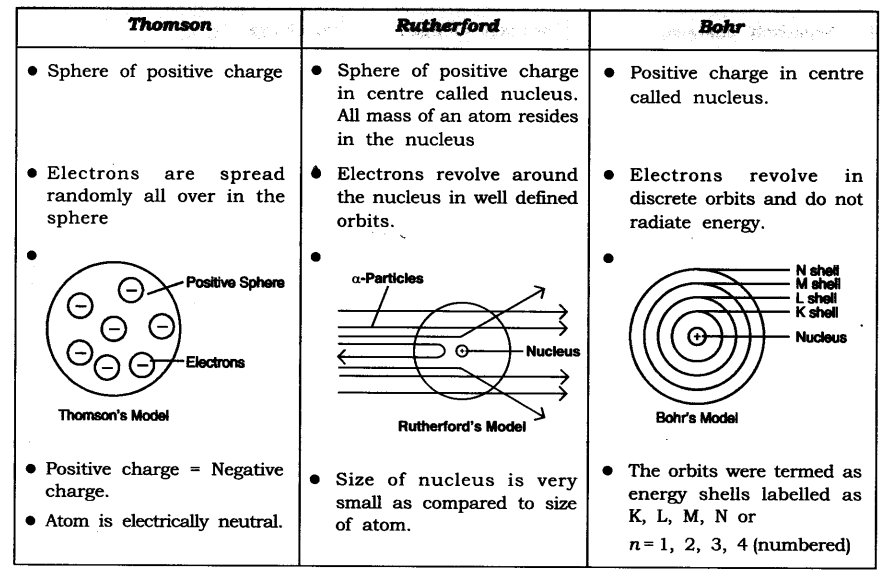
Question 6. Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Answer: The rules for writing of distribution of electrons in various shells for the first eighteen elements are:
(i) The maximum number of electrons present in a shell is given by the formula-2 n2
∵ n = orbit number i.e., 1, 2, 3
∵ Maximum number of electrons in different shells are:
K shell n = 1 2n2 => 2(1)2 = 2
L shell n = 2 2n2 => 2(2)2 = 8
M shell n = 3 2n2 => 2(3)2 = 18
N shell n = 4 2n2 => 2(4)2 = 32
(ii) The maximum number of electrons that can be accommodated in the outermost orbit is 8.
(iii) Electrons are not accommodated in a given shell unless the inner shells are filled. (Shells are filled step-wise).
Question 7. Define valency by taking examples of silicon and oxygen.
Answer: Valency is the combining capacity of an atom.
Atomic number of oxygen = 8 Atomic number of silicon = 14 K L M
Electronic configuration of oxygen = 2 6 –
Electronic configuration of silicon =2 8 4
In the atoms of oxygen the valence electrons are 6 (i.e., electrons in the outermost shell). To fill the orbit, 2 electrons are required. In the atom of silicon, the valence electrons are 4. To fill this orbit 4 electrons are required.
Hence, the combining capacity of oxygen is 2 and of silicon is 4.
i.e., Valency of oxygen = 2
Valency of silicon = 4
Question 8. Explain with examples
(i) Atomic number,
(ii) Mass number,
(iii) Isotopes and
(iv) Isobars.
Give any two uses of isotopes.
Solution:
(i) The number of positively charged protons present in the nucleus of an atom is defined as the atomic number and is denoted by Z. Example: Hydrogen has one proton in its nucleus, hence its atomic number is one.
(ii) The total number of protons and neutrons present in the nucleus of an atom is known as the mass number. It is denoted by A. 20Ca40 . Mass number is 40. Atomic number is 20.
(iii) The atoms which have the same number of protons but different number of neutrons are referred to as isotopes. Hence the mass number varies.
Example: The most simple example is the Carbon molecule which exists as 6C12 and 6C14
(iv) Isobars: Isobars are atoms which have the same mass number but differ in the atomic number.
Examples are, 20Ca40and 18Ar40
Uses of isotopes:
- The isotope of Iodine atom is used to treat goitre and iodine deficient disease.
- In the treatment of cancer, an isotope of cobalt is used.
- Fuel for nuclear reactors is derived from the isotopes of the Uranium atom.
Question 9. Na+ has completely filled K and L shells. Explain.
Answer: Sodium atom (Na), has atomic number =11
Number of protons =11
Number of electrons = 11
Electronic configuration of Na = K L M – 2 8 1
Sodium atom (Na) looses 1 electron to become stable and form Na+ ion. Hence it has completely filled K and L shells.
Question 10. If bromine atom is available in the form of, say, two isotopes 35Br79(49.7%) and 35Br81(50.3%), calculate the average atomic mass of Bromine atom.
Solution:
The atomic masses of two isotopic atoms are 79 (49.7%) and 81 (50.3%).
Thus, total mass = (79 * 49.7 / 100) + (81 * 50.3 / 100) = 39.263 + 40.743 = 80.006 u
Question 11. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 8X16and 8X18in the sample?
Solution:
Let the percentage of 8X16 be ‘a’ and that of 8X18 be ‘100-a’.
As per given data,
16.2u = 16 a / 100 + 18 (100-a) /100
1620 = 16a + 1800 – 18a
1620 = 1800 – 2a
a = 90%
Hence, the percentage of isotope in the sample 8X16 is 90% and that of
8X18 = 100-a = 100- 90=10%
Question 12. If Z=3, what would be the valency of the element? Also, name the element.
Solution:
Given: Atomic number, Z = 3
The electronic configuration of the element = K-2; L-1, hence its valency = 1
The element with atomic number 3 is Lithium.
Question 13. Composition of the nuclei of two atomic species X and Y are given as under
X Y
Protons = 6 6
Neutrons = 6 8
Give the mass numbers of X and Y. What is the relation between the two species?
Solution:
Mass number of X: Protons + neutrons = 6+6 = 12
Mass number of Y: Protons + neutrons = 6+8 = 14
They are the same element as their atomic numbers are the same.
They are isotopes as they differ in the number of neutrons and hence their mass numbers.
Question 14. For the following statements, write T for true and F for false.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Solution:
(a) Statement is False
(b) Statement is False
(c) Statement is True
(d) Statement is False
Question 15. Put a tick(✓) against correct choice and cross(x) against wrong choice in questions 15, 16 and 17.
Rutherford’s alpha – particle scattering experiment was responsible for the discovery of
(a) Atomic nucleus
(b) Electron
(c) Proton
(d) Neutron
Solution:
(a) Atomic nucleus
Question 16. Isotopes of an element have
(a) The same physical properties
(b) Different chemical properties
(c) Different number of neutrons
(d) Different atomic numbers.
Solution:
(c) Different number of neutrons
Question 17. Number of valence electrons in Cl– ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Solution:
(b) 8
Electronic distribution of Cl is K-2, L-8, M-7. Valence electrons are 7, hence chlorine gains one electron for the formation of Cl–. Therefore, its valency is 8.
Question 18. Which one of the following is a correct electronic configuration of Sodium?
(a) 2, 8
(b) 8, 2, 1
(c) 2, 1, 8
(d) 2, 8, 1
Solution:
(d) 2, 8, 1
Complete the following table.
| Atomic number | Mass number | Number of neutrons | Number of Protons | Number of electrons | Name of the atomic species |
| 9
16 – – – |
–
32 24 2 1 |
10
– – – 0 |
–
– 12 1 1 |
–
– – – 0 |
–
Sulphur – – – |
Solution:
The following table depicts the missing data:
Atomic number(Z) =Number of protons
Mass number = Number of neutrons + atomic number
(or)
Mass number(A) = Number of neutrons + number of neutrons
| Atomic number | Mass number | Number of neutrons | Number of Protons | Number of electrons | Name of the atomic species |
| 9
16 12 1 1 |
19
32 24 2 1 |
10
16 12 1 0 |
9
16 12 1 1 |
9
16 12 1 0 |
Fluorine
Sulphur Magnesium Deuterium Hydrogen |


